15 May 2006
Neuroscience Therapy Corp. (OTC: NPYC) has received FDA approval to begin marketing its electronic pain relief device the P-Stim in the United States.
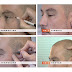
P-Stim, which the company claims has been highly successful in treating pain in thousands of patients in Europe, is now permitted to be marketed in the U.S. It is a miniaturized electro-stimulation device that is placed behind the ear with adhesive. It transmits low frequency electrical pulses via acupuncture-like needles inserted into the ear muscles. The electrical stimulation releases endorphins, which have an analgesic effect. The P-Stim can give continuous pain therapy over several days.
It was stated by the National Institute of Health: “Pain is a critical national health problem. It is the most common reason for medical appointments, nearly 40 million visits annually, and costs this country over $100 billion each year in health care and lost productivity.”
Neuroscience Therapy is now able to enter the multi-billion dollar market for pain releif in the U.S. and plans to begin a nationwide campaign to promote P-Stim.
President and CEO Dr. Randolph A. Turpin of Neuroscience Therapy said: “I’m very proud of our team for enabling Neuroscience Therapy to get to this point. We are now in an ideal position to begin making revenues in this multi-billion dollar market.” The company claims that its product has no known side effects, unlike many pharmaceutical pain relievers.
Home »
» Neuroscience Therapy Corp receives US approval to market pain-relief device
Neuroscience Therapy Corp receives US approval to market pain-relief device
If You Like product from here
Click Here free product informationGoogle Android PC Tablet






No comments:
Post a Comment